
- Home
- Research Navigation
-
Products
- Recombinant antibody
- Tag Nano-Antibody
- Recombinant Tag Antibody
- Nano-Secondary Antibody
- Recombinant Secondary Antibody
- For IP Nano-Secondary Antibody
- For WB Nano-Secondary Antibody
- Nanoselector
- Smart Ligands®
- Smart Booster
- SmartCapture® For Purification
- Flow Cytometry Antibody
- Recombinant VHH
- Antibody Biosimilar
-
Service
- Technical support
- About
- Blog
Application prospect of single-domain antibody in ADC drug
Foreword
Amid the wave of precision medicine, antibody-drug conjugates (ADC) have become a hot topic of research due to their ability to efficiently target tumor cells. However, their use in cancer treatment faces significant limitations due to acquired drug resistance and potential side effects. Single-domain antibody ADC (nADC) derived from VHH-target ADC offer superior stability, solubility, and manufacturability, enabling cost-effective production and expanding the range of targetable antigens. Therefore, nADC are promising candidates for next-generation ADC and may enhance the efficacy and safety of antibody-drug conjugates as a cancer treatment.
Antibody-drug conjugates (ADC)
ADC is composed of monoclonal antibodies targeting specific antigens and small molecule cytotoxic drugs linked through linkers, combining the powerful killing effects of traditional small molecule chemotherapies with the tumor-targeting properties of antibody drugs. However, the large size of conventional antibodies (mAb) hinders their ability to extravasate and effectively penetrate tissues, and the drug attached to the mAb tends to be unevenly distributed within the tumor. Therefore, ADC development must consider improving tissue permeability and minimizing off-target effects. Single-domain antibody (sdAb), as antibodies with unique properties, have shown great potential in the development of new-generation ADC.
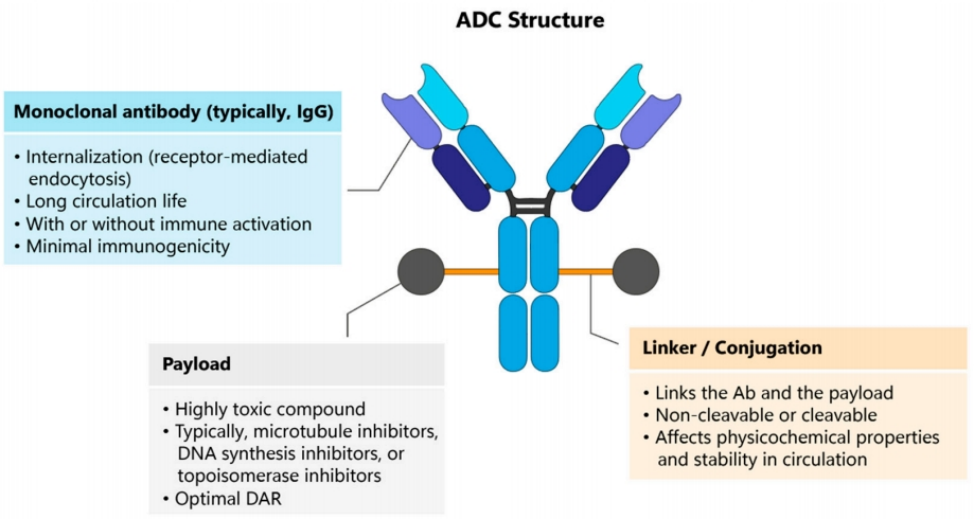
F1. The general structure of an ADC consists of three parts: the mAb, the payload, and a chemical linker between the payload and the antibody.
VHH and nADC Structure
HCAb is found in camelidae (Bactrians and camels, alpacas, and llamas) as well as cartilaginous fish (such as sharks). The VHH derived from Camelidae is a unique functional single domain of HCAb (Figure 2a). Although the molecular weight of the variable domain of the heavy chain in HCAbs is only one-tenth that of traditional IgG (12-17 kDa compared to 150 kDa), they retain high antigen-binding affinity and are the smallest naturally occurring antigen-binding fragments.
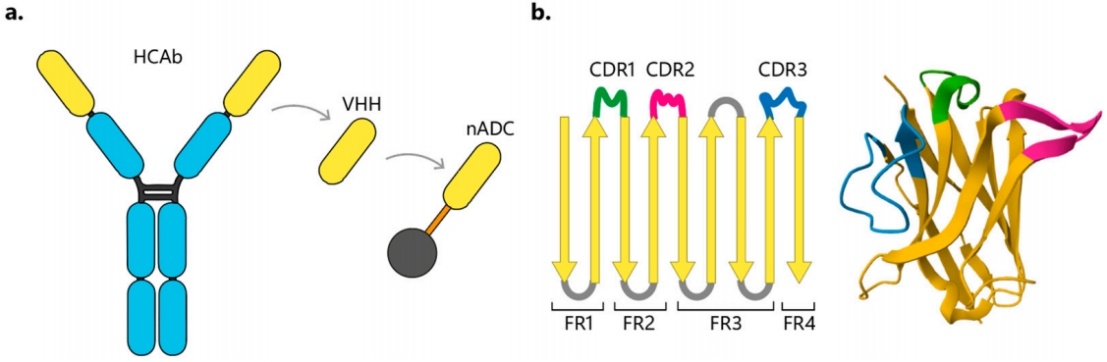
F2. Schematic diagrams of VHH and nADC structure. (a) Schematic diagrams of HCAb, VHH, and nADC. (b) Schematic diagram of the VHH structure and molecular 3D structure, showing the complementarity-determining regions (CDR) known to be responsible for antigen recognition.
VHH Biological Properties
VHH share many properties with mAb, allowing them to be conjugated to payloads. However, their simpler structure facilitates genetic modification without loss of affinity. Compared to mAb, VHH has a significant advantage in the generation of targeted ADC (Table 1).
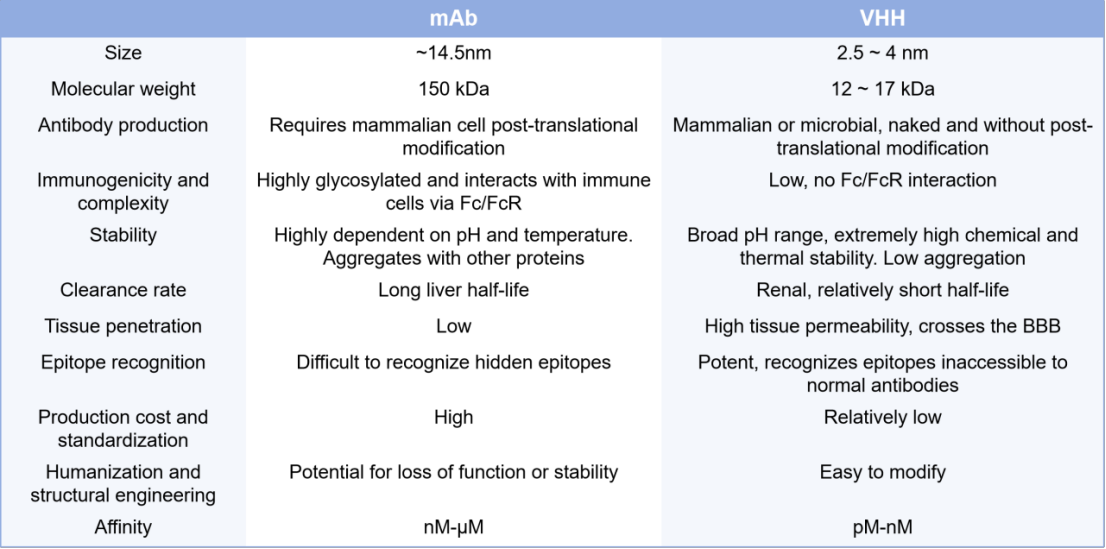
Table 1. Main differences between mAb and VHH
Advantages of VHH in developing novel ADC
1. Flexibility and Conjugation Methods
VHH can also be conjugated using lysine, cysteine, or site-specific conjugation. Compared to mAb, VHH is rich in amino acids such as lysine, aspartic acid, and glutamic acid, allowing for higher DARs through conjugation of these residues. The VHH structure and lack of complex post-translational modifications offer advantages over IgG antibodies in introducing non-natural amino acids or cysteines for site-specific conjugation. Site-specific conjugation is preferred because the presence of lysines in the CDR binding site can reduce affinity if conjugated via these sites. Introducing additional cysteines at positions distal to the antigen-binding site, preferably at the C-terminus, can partially address these issues.
In the clinical setting, unconjugated antibodies are generally well tolerated, allowing high-dose administration to target receptors on cell layers closer to blood vessels and penetrate deeper into tumor tissue. VHH reduces the size of the conjugate while maintaining affinity and specificity, helping it to enter solid tumors through blood vessels and significantly enhance its therapeutic effect. For example, in a patient-derived organoid (PDO) model, a comparative study of an anti-5T4 VHH-SN38 and a traditional anti-5T4-SN38 found that VHH exhibited superior penetration and greater tumor regression.
Furthermore, unlike mAb that may experience prolonged circulation time due to FcRn interaction, VHH do not interact with FcRn, ensuring their trafficking to lysosomes is unaffected, a property that can lead to more efficient release of the payload after lysosomal degradation.
2. Barrier-crossing transport capability
Delivering biologics to the central nervous system (CNS) presents significant challenges, primarily due to the multiple barriers between the CNS and the peripheral environment, including the blood-brain barrier (BBB), the blood-tumor barrier (BTB), and the binding site barrier (BSB). These barriers are highly selective and regulated, tightly controlling the exchange of substances between the blood and brain parenchyma. This challenge is particularly daunting for antibody-drug conjugates (ADC), as the mAb payloads they carry, modified in structure, size, and interaction with endothelial cells, may prematurely release the drug before reaching the target tumor, increasing the risk of neurotoxicity and leading to serious complications. In contrast, due to ability of VHH to penetrate tumors more rapidly than traditional antibodies and their stability in acidic environments, are ideal delivery vehicles for traversing these barriers and effectively reaching central tumor areas.
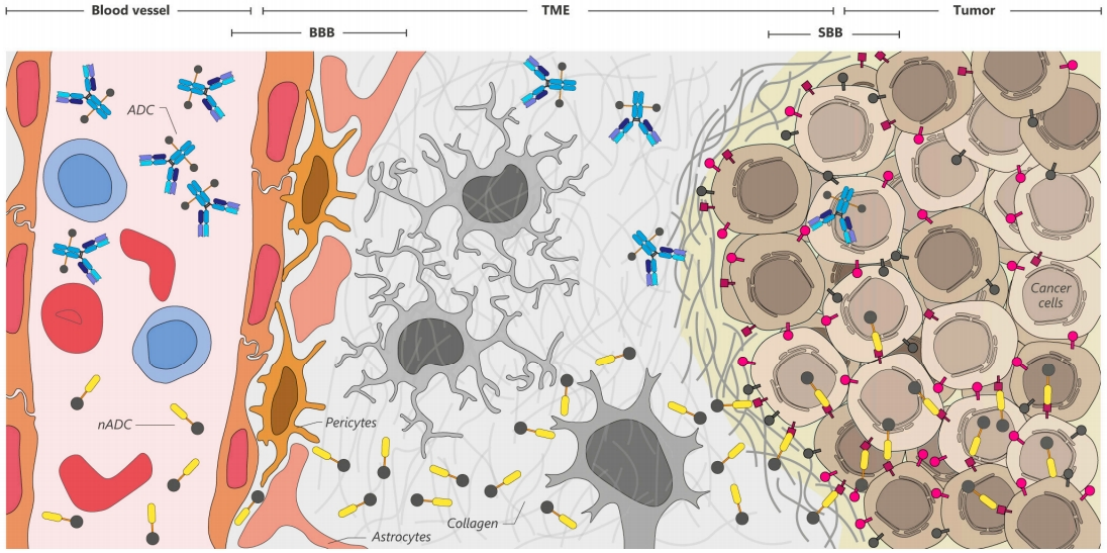
F 3. Transport of ADC and nADC across the BBB and BSB. The BBB restricts entry of substances from the bloodstream into the brain parenchyma, while the BSB restricts penetration of antibodies (mAb) and VHHs into tumors, resulting in uneven distribution of ADC and nADC. Compared with mAb-based ADC, nADC exhibit enhanced ability to cross the BBB and BSB, facilitating more efficient delivery to brain and tumor tissues. TEM: tumor microenvironment.
Development Progress of nADC
To date, no VHH-drug conjugates have entered clinical development, and all published studies remain in the preclinical phase. These studies have explored direct conjugation of nanobodies to drugs using a variety of formats and linkers. In some cases, VHHs have been combined with other agents for drug delivery. Major conjugated payloads include doxorubicin, MMAE, cisplatin, and SN38 (Table 2).
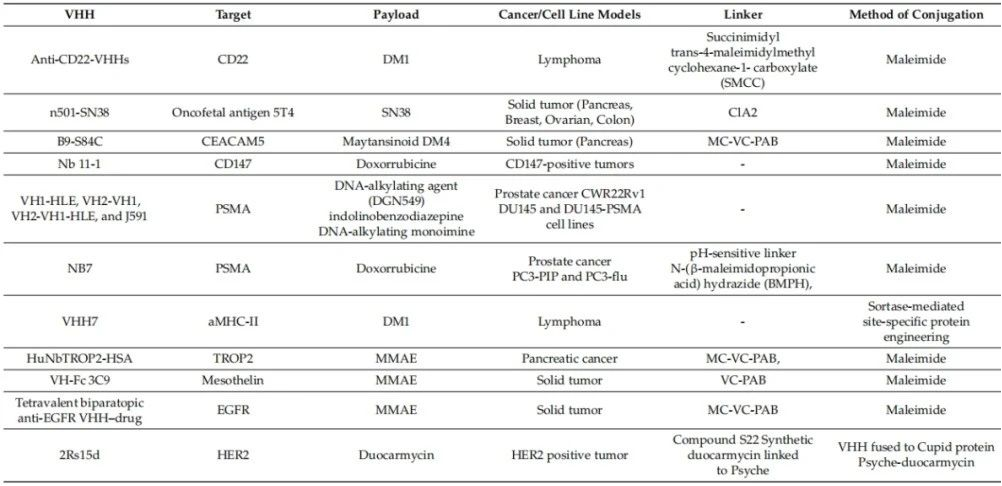
Table 2 Preclinical studies of VHH-drug conjugates
AlpVHHs® specializes in developing and producing customized, high-performance alpaca nanobodies. We provide tailored single-domain antibody (VHH or sdAb) pre-discovery CRO services and innovative nanobody products as the next-generation RUO tools for life science research. You can visit our website www.alpvhhs.com to learn more.

