
- Home
- Research Navigation
-
Products
- Recombinant antibody
- Tag Nano-Antibody
- Recombinant Tag Antibody
- Nano-Secondary Antibody
- Recombinant Secondary Antibody
- For IP Nano-Secondary Antibody
- For WB Nano-Secondary Antibody
- Nanoselector
- Smart Ligands®
- Smart Booster
- SmartCapture® For Purification
- Flow Cytometry Antibody
- Recombinant VHH
- Antibody Biosimilar
-
Service
- Technical support
- About
- Blog
Breakthrough in Phage Display: Ex-M13K07 Helper Phage Boosts VHH Antibody Discovery
Phage display system is widely used in antibody discovery, including VHH antibody (nanobody) development. The most commonly used approach is the monovalent display system based on M13 phage and its helper phage M13K07. However, to achieve monovalent display, the efficiency of phage display is relatively low. Reports suggest that the display efficiency is usually only around 10%. For library with a low proportion of specific clones, such as naïve or synthetic VHH antibody library, the screening efficiency becomes extremely limited.
In 2002, the Cha research team published an article in Nucleic Acids Research “An improved helper phage system for efficient isolation of specific antibody molecules in phage display”.
By introducing two amber stop codon mutations into the gIII gene of the helper phage, researchers created a situation where amber suppression occurs in suppressor strains such as TG1. As a result, expression of the PIII protein is significantly reduced. During phage assembly, the relative proportion of PIII proteins fused with antibody sequences (including scFv or VHH domains) on phagemid vectors becomes much higher, thereby enhancing the display efficiency.
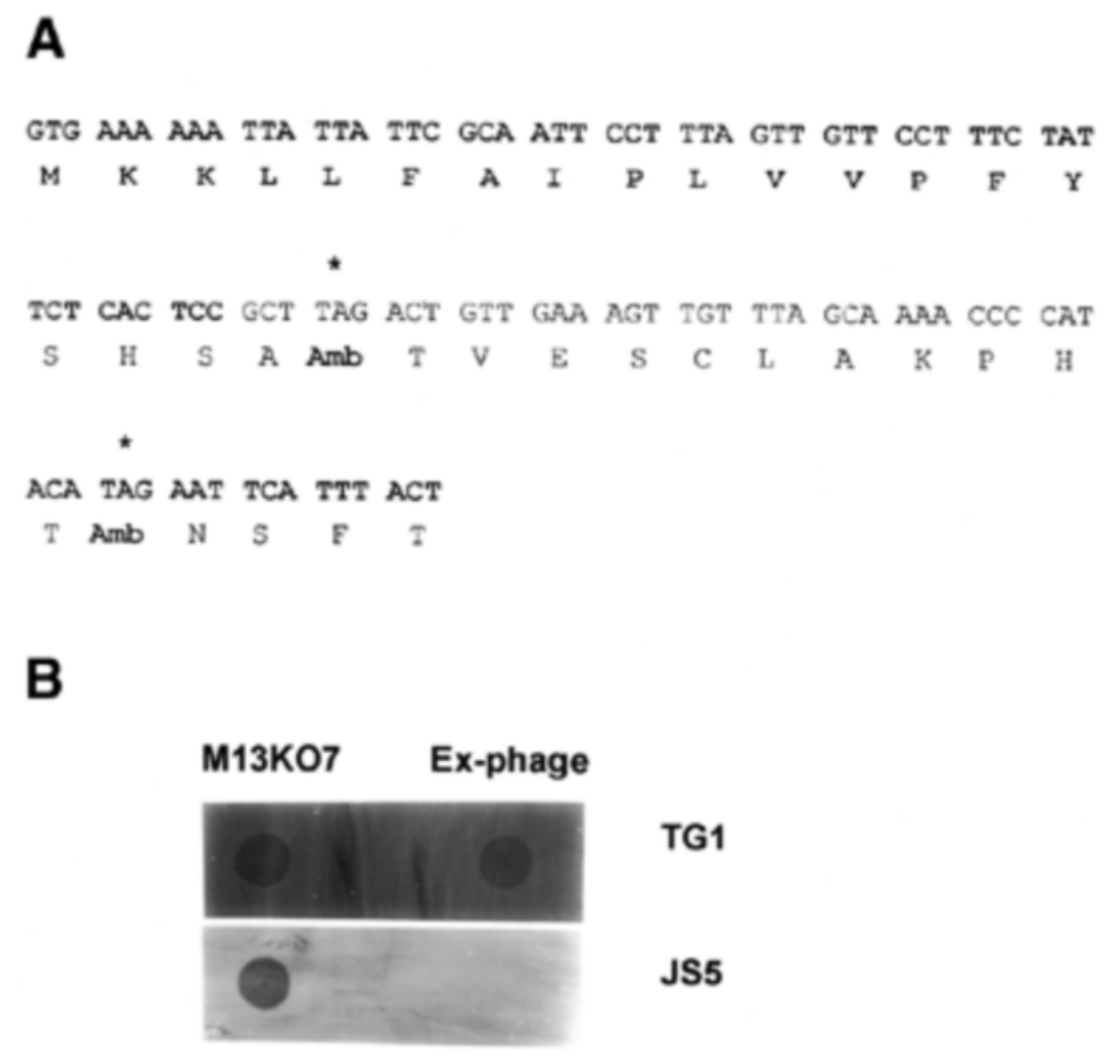
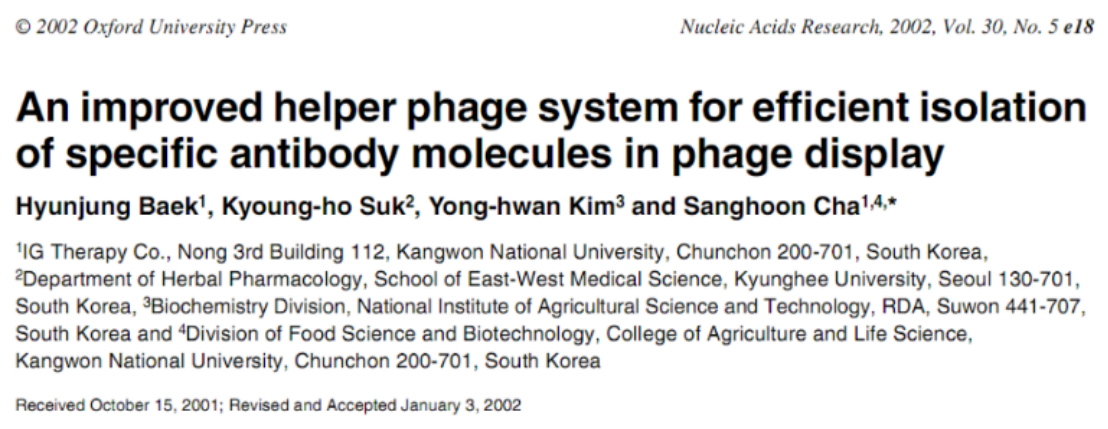
Figure 1. Modification sites of M13KO7 helper phage
A: Codon mutation sites.
B: Plaque assay analysis. Ex-phage particles could be detected in suppressor strains, whereas in non-suppressor strains, gIII protein could not be expressed, preventing phage packaging.
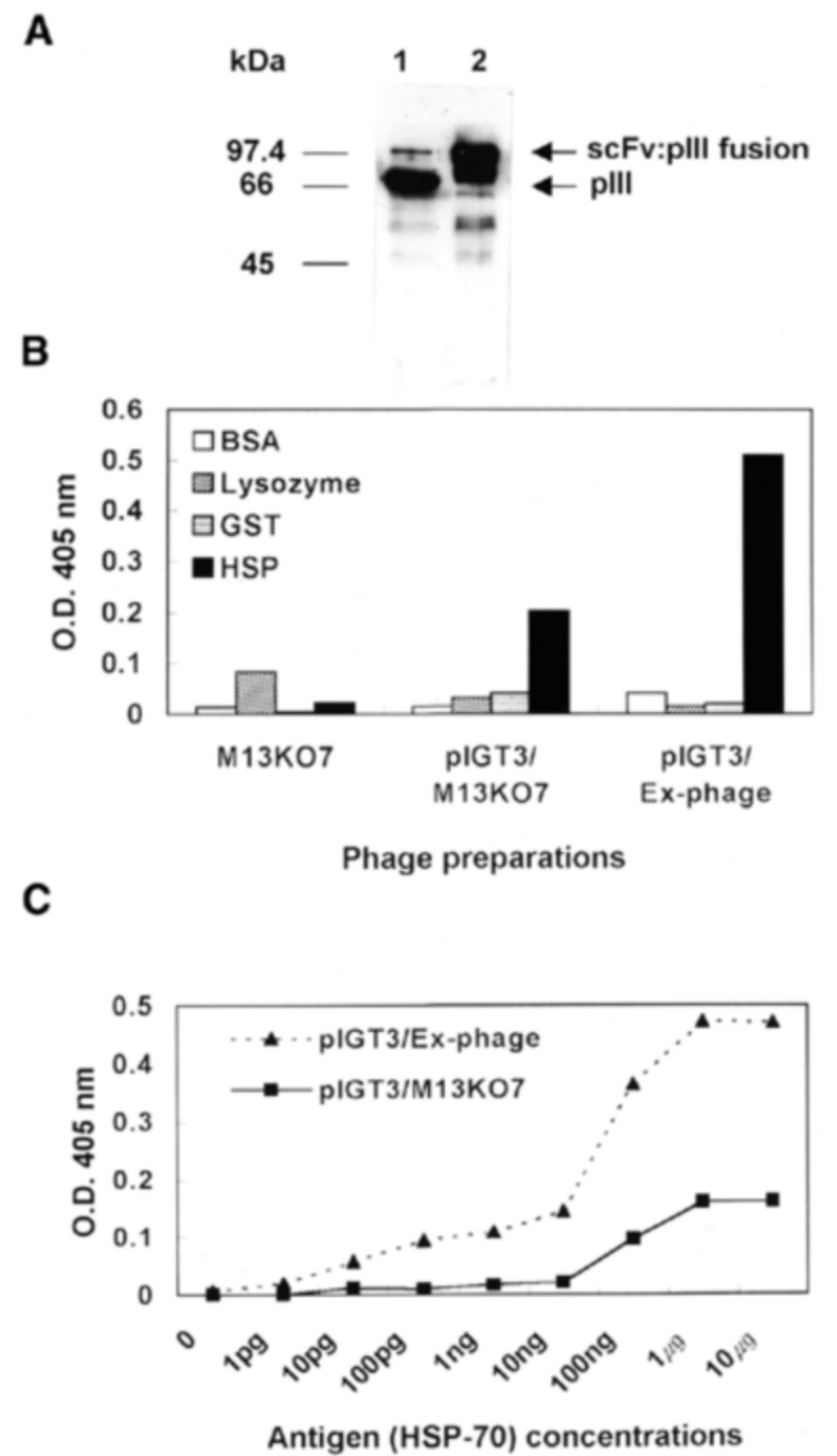
Figure 2. Analysis of the efficiency of single-chain antibody display using Ex-M13KO7 helper phage.
A: Lanes 1 and 2 show protein extracted from phage particles rescued by M13KO7 and Ex-M13KO7, respectively. Identification using an anti-p3 antibody shows that lane 2 contains a higher level of p3 protein, indicating significantly improved display efficiency.
B: ELISA plates coated with different proteins and tested with phage particles rescued by different helper phages.
C: ELISA plates coated with HSP-70 antigen and tested with phage particles rescued by different helper phages, demonstrating improved sensitivity due to multivalent display.
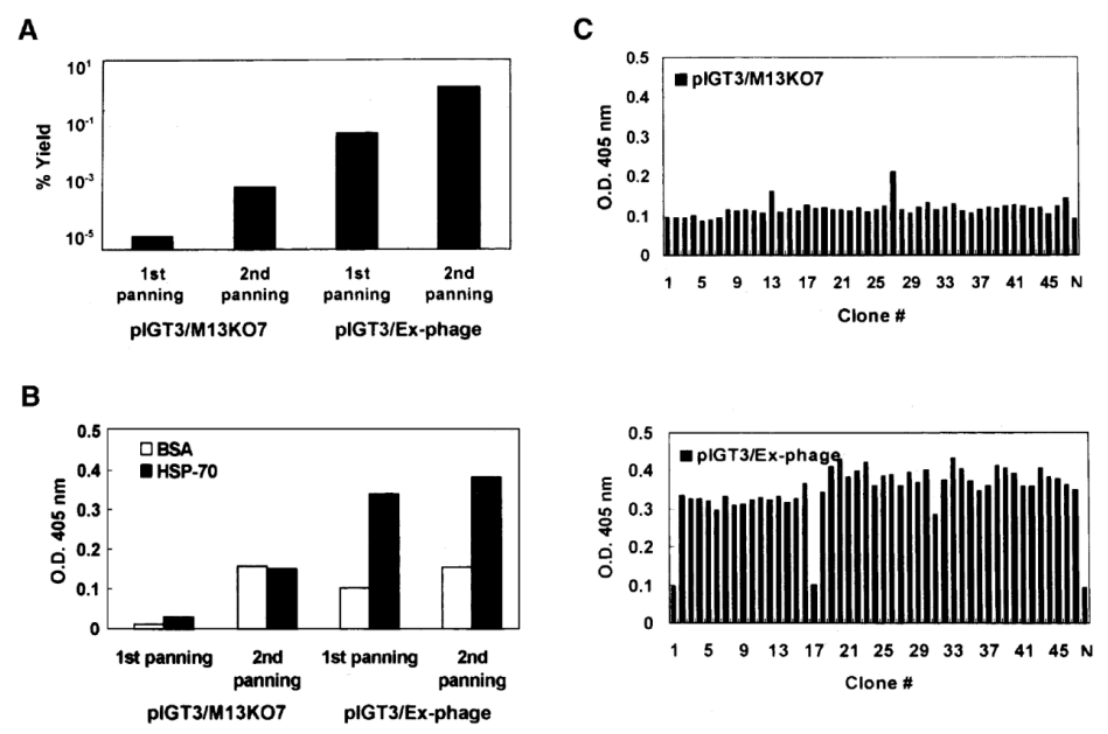
Figure 3. Comparison of two helper phage screening systems.
Phage-ELISA shows that the Ex-phage system has more significant enrichment.
The purpose of the first round of screening of phage display library is to enrich as many positive clones as possible and retain as much diversity as possible, so affinity issues do not need to be considered at this stage. For natural and synthetic libraries, the proportion of specific clones is low. In traditional phage display, the efficiency of antibody display on the surface of phage particles is low, which makes it very difficult and clones are easily lost. By modifying the helper phage to significantly improve the display efficiency of antibodies on the surface of phage particles, the positive rate can be significantly increased and the clone loss rate can be reduced. Ex-M13K07 is a helper phage that can significantly improve the display efficiency and can be used for phage library packaging and panning processes.
Recommendations for Use:
1. For naïve and synthetic library (e.g., Naïve VHH Library), Ex-M13K07 is recommended for packaging and screening. Through multivalent display and enhanced efficiency, more positive clones can be enriched.
2. For immune library, Ex-M13K07 is useful for initial library packaging, while M13K07 monovalent display is recommended in subsequent rounds to facilitate isolation of higher-affinity clones.
3. When performing selections against cell surface targets with low expression levels, Ex-M13K07 is particularly advantageous. It is also suitable for cell-based assays such as phage-ELISA or Phage-FACS using VHH or scFv library.
AlpVHHs provides Ex-M13K07 to improve phage display library efficiency:
Code | Description | Applications | Size |
P013 | Phage Display | 1mL |
This innovative helper phage provides researchers with a reliable and efficient tool for advancing antibody discovery pipelines, from scFvs to next-generation VHH nanobody.
Reference:
Baek H, Suk K, Kim Y, et al. An improved helper phage system for efficient isolation of specific antibody molecules in phage display[J]. Nucleic acids research, 2002, 30(5): e18-e18.

