
- Home
- Research Navigation
-
Products
- Recombinant antibody
- Recombinant Primary Antibody
- Tag Nano-Antibody
- Recombinant Tag Antibody
- Nano-Secondary Antibody
- Recombinant Secondary Antibody
- For IP Nano-Secondary Antibody
- For WB Nano-Secondary Antibody
- Nanoselector®
- Smart Ligands®
- SmartCapture® For Purification
- Smart Booster
- Flow Cytometry Antibody
- Recombinant VHH
- Antibody Biosimilar
-
Service
- Technical support
- About
- Blog
IP/Co-IP Series – (II) Experimental Design of IP/Co-IP
AlpVHHs will soon publish a special series on IP/Co-IP to help researchers master immunoprecipitation (IP) and co-immunoprecipitation (Co-IP) techniques, and to solve common experimental problems. This issue focuses on how to design an IP/Co-IP experiment. Many researchers are often confused about what kinds of control groups are necessary. A rigorous experimental design is the cornerstone of reliable results!
When designing an IP/Co-IP experiment, antibody selection is critical. The quality, specificity, and reproducibility of the antibody directly determine the success of the assay. In recent years, recombinant antibody production has provided researchers with a powerful alternative to traditional hybridoma-derived antibodies. Recombinant antibodies, including VHH antibodies and nanobodies, offer several advantages — such as higher consistency between batches, customizable affinity, and better stability under diverse experimental conditions.
Today, let’s discuss how to design a logically sound IP/Co-IP experiment.
What Are Endogenous and Exogenous IP/Co-IP?
In IP/Co-IP experiments, based on the source of the target protein, experiments can generally be divided into endogenous and exogenous types.
Endogenous IP/Co-IP
● Purpose:
To study proteins naturally present in cells or the interactions between them.
● Features:
No genetic engineering is required.
Reflects the most physiologically relevant state of the protein.
Requires antibodies with high specificity and affinity.
Exogenous IP/Co-IP
● Purpose:
To study recombinant proteins introduced into cells via transfection — typically tagged with epitopes such as Flag or HA — focusing on their expression, modification, or interactions.
● Features:
High enrichment efficiency and specificity due to tag-based purification.
Clearer background signals.
Widely used for validating specific protein–protein interactions.
Controls for Endogenous Protein Experiments
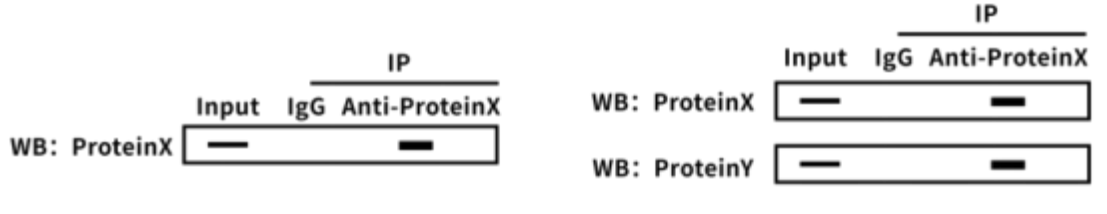
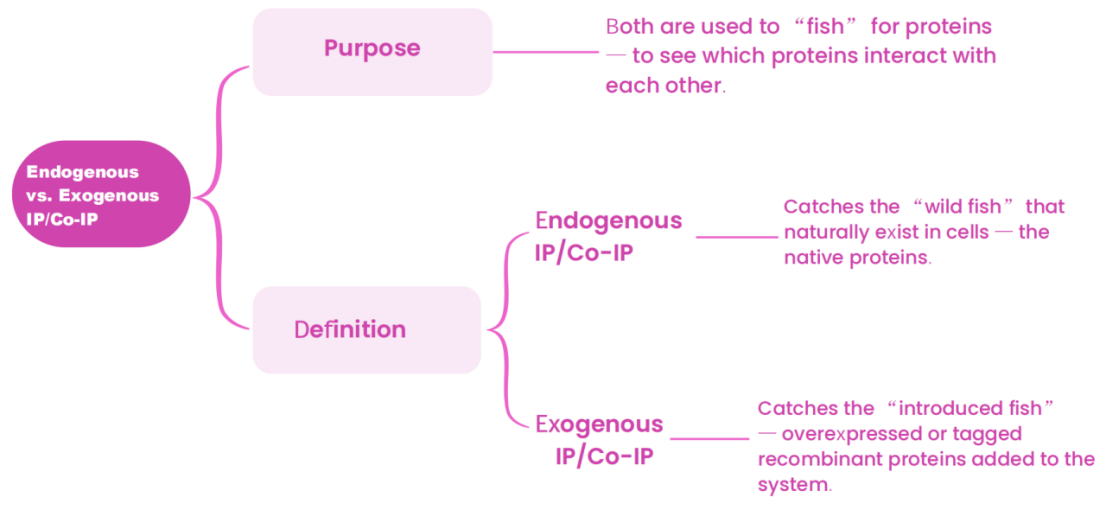
Figure 1: Schematic diagram of endogenous IP experiment results
● Input Control (Positive Control) – untreated lysate
Purpose: Confirm that the target protein is expressed in the sample, and rule out false negatives caused by lysis failure or protein degradation.
● IgG Control (Negative Control) – same-species IgG
Purpose: Eliminate false positives due to nonspecific antibody binding.
● IP Experimental Group
Purpose: Use a specific antibody to verify successful enrichment of the target protein and to detect whether two proteins interact.
● Optional Control: Flow-through Detection
Purpose: Collect the supernatant after IP to assess the efficiency of immunoprecipitation.
Controls for Exogenous Protein Experiments
Figure 2: Schematic diagram of exogenous IP experiment results
● Input Control (Positive Control)
Purpose: Verify the transfection efficiency of exogenous proteins and confirm that interacting proteins are co-expressed in cells.
● Untransfected / Single Transfection Control (Negative Control)
Purpose: Eliminate interference from endogenous proteins or nonspecific binding that could lead to false positives.
● IP Experimental Group
Purpose: Use tag-specific beads to confirm successful enrichment of the exogenous protein and to test for its interaction with the target protein.
● Optional Control: Flow-through Detection
Purpose: Collect the supernatant after IP to evaluate enrichment efficiency.
When designing an IP/Co-IP experiment, selecting proper control groups (such as Input, IgG negative control, untransfected control, etc.) is fundamental to ensure result reliability. In addition, AlpVHHs has developed a suite of recombinant nanobodies (VHHs) and nano-secondary antibodies, which can offer the following advantages in IP/Co-IP workflows:
● Nanoselector Series (Universal IP/Co-IP Beads Bestseller)
Stable Capture: Nanobody-based beads with picomolar-level affinity ensure ultra-strong and specific target binding.
High Capacity: Over 4× higher binding capacity compared to conventional agarose or magnetic beads.
Fast Binding: Complete incubation in just 0.5–1 hour at 4 °C.
No Heavy/Light Chain Interference: Designed for tag-protein IP/Co-IP — completely eliminates antibody chain contamination in downstream detection.
● For-IP Nano-Secondary Antibody (IP-Dedicated Nano-Secondary)
Avoid Light/Heavy Chain Interference: Specifically recognizes either the light or heavy chain region, bypassing chain-derived background in WB after IP.
Species-Independent: Compatible with mouse and rabbit primaries — one reagent for all.
Time-Saving: Requires only 1 hour of incubation for clean, high-specificity results.
● Recombinant Tag Antibody (HRP tag Antibody)
No Secondary Required: HRP-labeled primary antibody — eliminates the need for secondary antibodies in WB.
High Affinity & Specificity: Achieves clear, low-background signals with just 1 hour of incubation.
Ultra-Clean Background: Optimized for direct detection of tag proteins with minimal nonspecific noise.

