
- Home
- Research Navigation
-
Products
- Recombinant antibody
- Tag Nano-Antibody
- Recombinant Tag Antibody
- Nano-Secondary Antibody
- Recombinant Secondary Antibody
- For IP Nano-Secondary Antibody
- For WB Nano-Secondary Antibody
- Nanoselector
- Smart Ligands®
- Smart Booster
- SmartCapture® For Purification
- Flow Cytometry Antibody
- Recombinant VHH
- Antibody Biosimilar
-
Service
- Technical support
- About
- Blog
Rapid In Vitro Cytotoxicity Testing Methods for Dual-Toxin ADC
Foreword
In the current upsurge of ADC drug development, differentiated competition has become the key to stand out. Although ADC technology has undergone many iterations, many ADC with single payloads still face dose limitations and less-than-expected effects in clinical studies. For example, the tubulin inhibitor MMAE has a weak effect on quiescent tumor cells and is prone to drug resistance. These problems may be related to the heterogeneity of tumor tissues, the resistance of ADC drugs, and the differences in toxin mechanisms. Resistance mechanisms involve reduced antigen expression, decreased ADC transport and processing capacity, resistance to cytotoxic payloads, and increased outflow of drugs from cells.
The study found that by precisely controlling the ratio of the two drugs and delivering two synergistic payloads to cancer cells, more effective therapeutic effects can be achieved, and with the application of two payloads with different mechanisms, the incidence of drug resistance will be significantly reduced. Based on the complementary mechanism of action between different toxins, the development of dual-load ADC drugs with improved synergistic efficacy may become a hot spot in the development of next-generation ADC drugs and the key to differentiated competition.
Advantages of dual-toxin ADC
1. Enhanced anti-tumor efficacy
⚪ Multi-mechanism effect: By combining two toxins with different mechanisms, multiple key pathways of tumor cells are destroyed at the same time, enhancing the killing effect.
⚪ Synergistic effect: The two toxins may produce a synergistic effect, further improving the efficacy.
2. Overcoming drug resistance
⚪ Reduce the risk of drug resistance: Tumor cells are prone to resistance to a single toxin, and the dual toxin design reduces the risk of drug resistance through multiple mechanisms.
⚪ Multiple strikes: Even if one mechanism fails, another mechanism can still work.
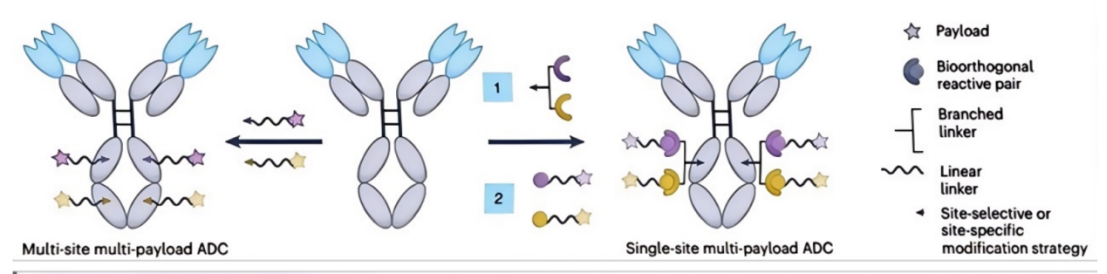
There have been some remarkable results in the study of dual-load ADC. For example, Levengood et al. prepared an ADC containing two different tubulin polymerization inhibitors (MMAE and MMAF), coupled MMAE and MMAF to CD30 antibodies, exerted complementary anticancer activities, and showed significant therapeutic effects in a single-drug resistant anaplastic large cell lymphoma xenograft model. These results show that the use of two payloads that target microtubules but have different mechanisms of action can potentially overcome treatment resistance and tumor recurrence caused by cell heterogeneity (multiple cell cycle states).
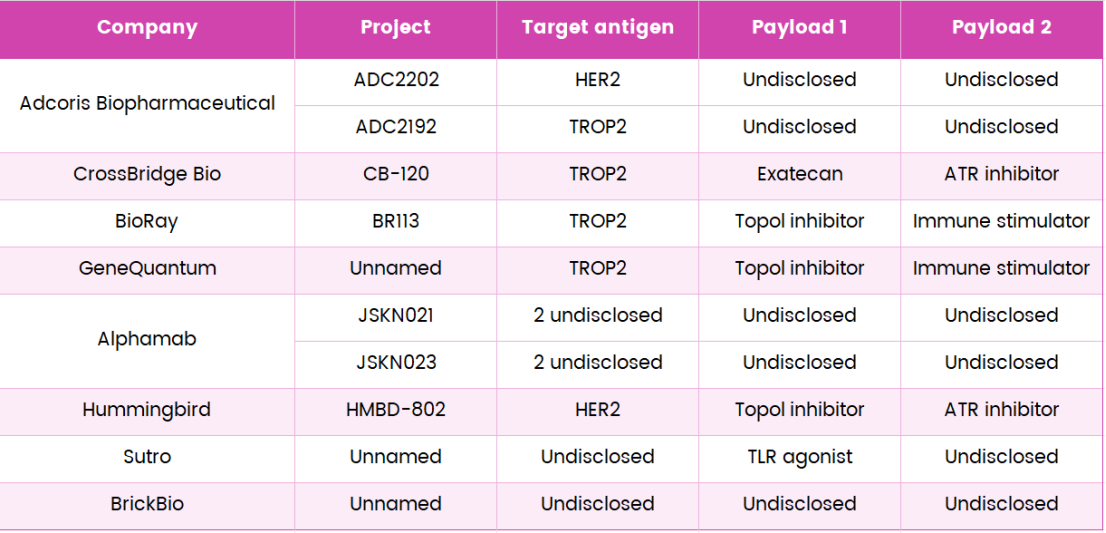
Not all studies of dual-payload ADC have demonstrated synergistic effects, especially those involving different payload classes. The importance of selecting payloads with appropriate mechanisms ensures balanced potency between the two selected payloads and optimizes the DAR value to achieve the best therapeutic effect.
At present, there is a lack of detection methods for the in vitro killing detection of dual toxin ADC, which requires coupling before detection, and the process is complex and the cycle is long. For this reason, AlpVHHs has carried out site-specific coupling of nanoantibodies that recognize different positions in the constant region of antibodies and developed standardized in vitro killing reagents to facilitate in vitro assembly and rapid screening of toxins and DAR.
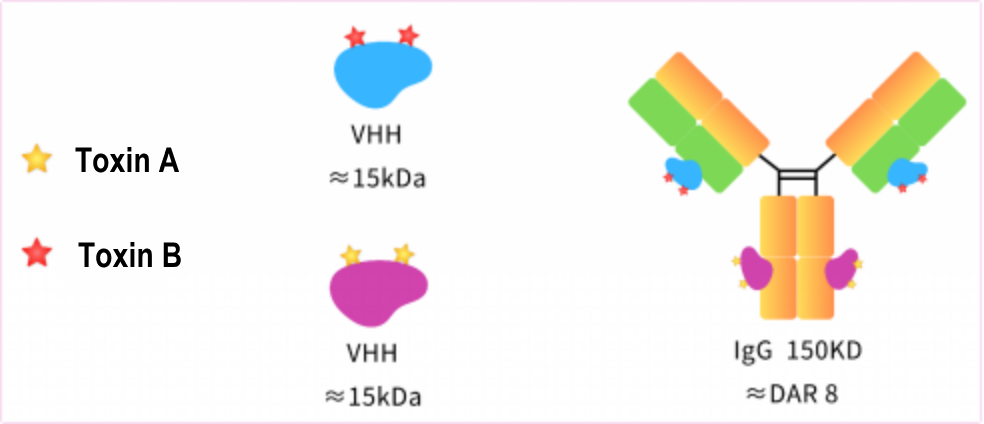
Product List
Anti-Human nano -2°ADC
Code | Description | Applications | Size |
023-102-101 | Internalization Test | 100 ug | |
023-101-101 | Internalization Test | 100 ug | |
023-102-102 | Internalization Test | 100 ug | |
023-101-102 | Internalization Test | 100 ug | |
023-102-103 | Internalization Test | 100 ug | |
023-101-103 | Internalization Test | 100 ug | |
023-102-104 | Internalization Test | 100 ug | |
023-101-104 | Internalization Test | 100 ug |
Anti-Mouse nano -2°ADC
Code | Description | Applications | Size |
001-106-101 | Internalization Test | 100 ug | |
001-101-102 | Internalization Test | 100 ug | |
001-106-102 | Internalization Test | 100 ug | |
001-101-101 | Internalization Test | 100 ug | |
001-106-103 | Internalization Test | 100 ug | |
001-101-103 | Internalization Test | 100 ug | |
001-106-104 | Internalization Test | 100 ug | |
001-101-104 | Internalization Test | 100 ug |

