
- Home
- Research Navigation
-
Products
- Recombinant antibody
- Recombinant Primary Antibody
- Tag Nano-Antibody
- Recombinant Tag Antibody
- Nano-Secondary Antibody
- Recombinant Secondary Antibody
- For IP Nano-Secondary Antibody
- For WB Nano-Secondary Antibody
- Nanoselector®
- Smart Ligands®
- SmartCapture® For Purification
- Smart Booster
- Flow Cytometry Antibody
- Recombinant VHH
- Antibody Biosimilar
-
Service
- Technical support
- About
- Blog
IP/Co-IP Series - (III) Sample Processing of IP/Co-IP
Foreword
AlpVHHs will be publishing a special column on IP/Co-IP to help researchers fully master IP/Co-IP experimental skills and improve applications using recombinant antibodies, particularly VHH antibodies (nanobodies) known for their high solubility and binding precision.
Due to the differences between samples from different sources (such as tissues and cells), many researchers often struggle with how to effectively process samples when preparing IP/Co-IP samples. This step is crucial to ensuring reliable subsequent results! If lysis is insufficient or inappropriate, even if the antibody can capture the target protein, a complete natural interacting complex cannot be obtained, ultimately leading to experimental failure. Today, we'll discuss how to "tailor-make" a lysis protocol for your samples.
Cell Sample Processing
1. Washing: Collect approximately 2 × 10⁷ cells and wash twice with pre-chilled 1 mL PBS, aspirating as much PBS as possible.
2. Lysis: Add 990 μL IP Lysis Buffer and 10 μL protease inhibitor cocktail (1%), vortex to mix.
3. Sonication: Sonicate for 3-5 min using an ultrasonic cell disruptor at 80 Hz, with 3-second sonication followed by 3-second pauses, then sonicate on ice.
4. Centrifugation: Centrifuge at 12000 rpm for 10 min at 4 °C. Collect the supernatant as total protein. Measure protein concentration and store at -80 °C for later use.
5. Input: Collect 1/10 of the total protein as input.
✳ Note: Protein extraction should be performed on ice throughout to minimize protein degradation. If detecting phosphorylated proteins, a phosphatase inhibitor mixture should be added. Sample volume ≥ 2 × 10⁷ cells.
Animal Tissue Sample Processing
1. Fresh Tissue Acquisition and Preservation: Quickly remove the target tissue, rinse thoroughly with pre-chilled PBS or physiological saline to remove blood and impurities, blot dry with filter paper, and then flash-freeze in liquid nitrogen at -80°C for long-term storage.
2. Grinding: Grind the frozen tissue into a fine powder in a pre-chilled liquid nitrogen mortar. For softer tissues such as liver and brain, place in a pre-chilled homogenizer tube and homogenize briefly and intermittently using an electric homogenizer.
3. Lysis: Add 990 μL of Lysis Buffer and 1% protease inhibitor cocktail to the mortar, grind on ice for 5-10 minutes until homogenized, and transfer to an EP tube.
4. Thorough Lysis: Lyse on ice for 30 minutes, inverting the tube every 5 minutes to mix.
5. Sonication: Sonicate for 8-10 minutes at 80 Hz, with 3-second sonication intervals followed by 3-second intervals, in an ice bath.
6. Centrifugation: Centrifuge at 12000 rpm for 10 minutes at 4℃. Collect the supernatant, which is the total protein. Measure the protein concentration and store at -80℃ for later use.
7. Input: Take 1/10 of the total protein as the input.
✳ Note: Do not repeatedly freeze and thaw. It is best to process fresh or aliquot into one batch; sample volume > 0.5g.
Plant Tissue Sample Processing
1. Collection and Grinding: Collect fresh or frozen plant samples and grind them thoroughly using a mortar and pestle or tissue homogenizer. Transfer the homogenate to a 50mL centrifuge tube and operate on ice for no more than 20 minutes.
2. Lysis: Add lysis buffer and a 1% protease inhibitor cocktail at a ratio of 100-200μL per 20mg of plant tissue. Adjust the amount of lysis buffer as needed based on the lysis progress.
3. Centrifugation: Centrifuge at 10000-14000g for 3-5 minutes at 4℃. Collect the supernatant as total protein. Measure the protein concentration and store at -80℃ for later use.
4. Input: Collect 1/10 of the total protein as input.
✳ Note: Ensure the sample is fresh and uncontaminated. Perform protein extraction on ice throughout to minimize protein degradation. If detecting phosphorylated proteins, add a phosphatase inhibitor mixture to the extraction buffer. Sample volume >2g.
Buffer formulation
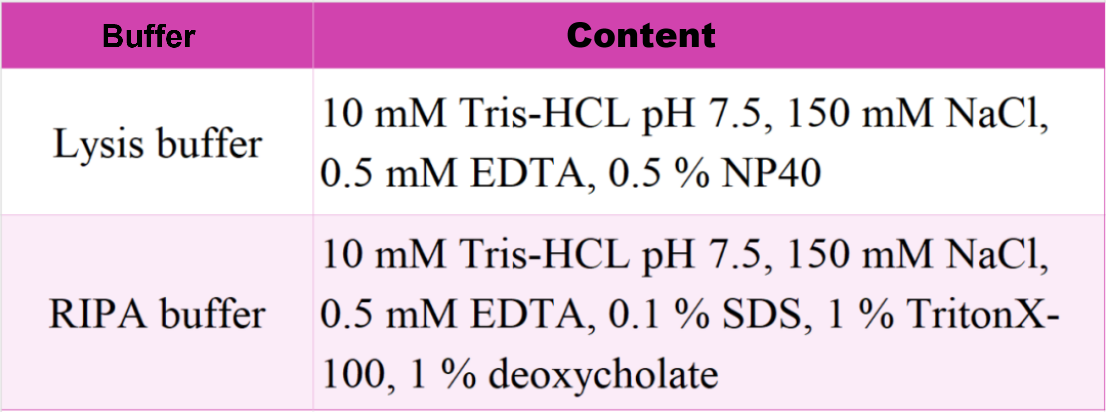
Choosing a Lysis Buffer
1. For cytoplasmic proteins, resuspend cell clumps in pre-chilled Lysis buffer by pipetting. Note: A protease inhibitor needs to be added to the Lysis buffer before use.
2. For nuclear/chromatin proteins, resuspend cell clumps in pre-chilled RIPA buffer. Note: DNase I (75-150 Kunitz U/mL), MgCl₂ (2.5 mM), and a protease inhibitor (1 mM) need to be added to the RIPA buffer before use.
3. For other cell types such as yeast, plants, insects, and bacteria, please use the appropriate cell lysis buffer.
This protocol is compatible with conventional IgG antibodies as well as recombinant antibodies, VHH antibodies, and nanobody-based IP/Co-IP workflows, supporting high sensitivity and stable protein complex detection. To learn more details, please visit our website: https://www.alpvhhs.com.

